edit checks in clinical data management ppt|Clinical Data Management: eCRF & Edit Checks : Clark Explore the critical concepts of data validation and edit checks in clinical data management. Learn how data validation techniques, including range checks, . Ass Videos and Anal Movies. All you booty lovers out there will enjoy this site, 100% guaranteed. We offer free streaming porn tubes, all related to asses ;) . Ebony Big Ass No video available 667K. Anal Sex In Homemade No video available 2.02M. BBW No video available 9.59M. Mom No video available 1.82M .
PH0 · Tips for Writing Effective Edit Checks in eCRFs
PH1 · Practical Guide to Clinical Data Management
PH2 · Ensuring Data Quality: A Comprehensive Guide to Edit Check
PH3 · Ensuring Data Quality: A Comprehensive Guide to Edit Check
PH4 · Enhancing Data Integrity by Applying Edit Checks to Your TMF
PH5 · Edit Checks in Clinical Data Management
PH6 · Edit Checks in Clinical Data Management
PH7 · Edit Checks
PH8 · Edit Check Design Principles
PH9 · Data Validation and Edit Checks
PH10 · Clinical Data Management: eCRF & Edit Checks
PH11 · Clinical Data Management
Because the Horses are an icon of authority, endurance and perseverance, they are a highly regarded auspicious symbol in Feng Shui, allowing the beholder to sprint stably through any obstacle in the journey of life. In Feng Shui, the horse, is a popular figure to display or hang in the home. Paintings and figurines can often be found placed .AutomotiveMotorcycleSLAPrimaryRechargeable
edit checks in clinical data management ppt*******Mahesh Koppula. An brief introduction to the clinical data management process is described in this slides. These slides provides you the information regarding .Most edit checks will be run automatically by the clinical data management (CDM) or EDC system being used for the study. A few checks will be performed manually, and others will be run outside of the .
By applying clinical data management type techniques similar to what we use in EDC, we can run logical checks (going forward, referred to as TMF edit checks), on our eTMF .edit checks in clinical data management ppt Clinical Data Management: eCRF & Edit Checks Explore the critical concepts of data validation and edit checks in clinical data management. Learn how data validation techniques, including range checks, .
Edit checks are a great mechanism to improve data quality within an EDC system. Learn how to write effective edit checks in clinical data management. - 2024This chapter discusses the use of edit checks in clinical studies, including the purpose of edit checks, types of edit checks, creation processes of edit check specifications and .
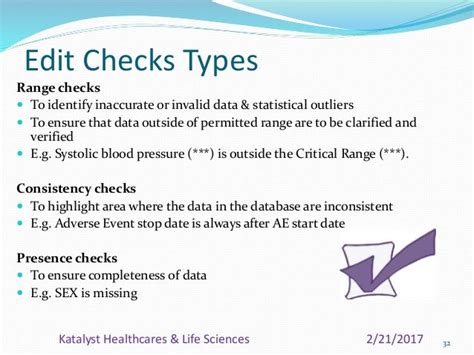
Edit checks are a great mechanism to improve data quality within an electronic data capture (EDC) system.There are many benefits to creating them in .
Ensuring data quality: Edit checks help maintain the accuracy and consistency of clinical trial data by automatically identifying and flagging errors or inconsistencies during data . The Data Management Plan. Abstract. Get Access. chapter | pages. CRF Design Considerations. Abstract. Get Access. chapter | pages. Database Design .Our Data Management Team informs our clinic of the edit checks implemented for each protocol. Our Statisticians and Data Managers communicate closely to ensure the proper edit checks are in place. This . While EDC edit checks can work well for simple data entry issues, more advanced checks are still needed to ensure overall data quality. . We have presented an integrated solution for clinical data review that helps data managers identify missing, erroneous and inconsistent data, manage queries and organize their workload in a .
Explore the critical concepts of data validation and edit checks in clinical data management. Learn how data validation techniques, including range checks, consistency checks, and logic checks, ensure accurate and reliable clinical trial data. Discover the role of automated edit checks, quality control, and regulatory compliance in maintaining high .PowerPoint Presentation. An introduction to CDISC and CDASH. Bring on the Standards! Emmanuelle Denis M.Sc., MICR. Global Health Clinical Trials Research Programme. * * * * Original - Original data or accurate transcription of original Attributable - Data records must indicate who recorded the information. Data must remain under investigator .
In clinical trial, one of the most important tasks facing clinical data management personnel is to produce the electronic Edit Checks specifications for a study. Developing the electronic edit check specification -- and processing the queries that result from them -- is arguably the most vital and time-consuming data cleaning activity data .
Clinical data management (CDM) consists of various activities involving the handling of data or information that is outlined in the protocol to be collected/analyzed. CDM is a multidisciplinary activity. This module will provide an overview of clinical data management and introduce the CCR’s clinical research database.
edit checks in clinical data management ppt Data validation: edit checks, source data verification, and data anonymization Experts in charge: data manager, database designer, quality control associate Clinical data validation is a series of quality tests to ensure accuracy, consistency, legibility, and integrity of information. This includes the following steps. . sopi_1234. This document discusses clinical data management (CDM) systems and processes. It defines key terms like source data, source documents, and raw data. It then describes the essential steps in CDM including initial planning, data collection, review and verification, coding, query resolution, data entry and validation, output and .
Constraints, or edit checks, are used to check data to make sure it meets standards/requirements and alerts the user that data entry fields have or may have errors, such as: Required data is missing. Entered data is not formatted correctly. Entered data does not meet range or order requirements. This allows data to be “cleaned” as it is . Clinical Data Management Overview. Andrew Taylor (安 泰乐 ) , M.S. Head of Clinical Data Management August 30, 2010. Learning Objectives. Overview of Process Related to Clinical Data Principles of Data Management Data Management Activities Changes Happening Now and in the Future.Clinical Data Management (CDM) processes, including Edit Checks could be applied to eTMF management and oversight Employing Edit Checks would potentially result in increased TMF compliance, greater quality compliance, and would be more efficient than our current, manual oversight processes Application of CDM processes could transform eTMF 032-2008: Clinical-Data Acceptance Testing Procedure Clinical-Data Acceptance Testing Procedure Sunil Gupta, Quintiles, Thousand Oaks, CA easy to update and facilitates good communication with the Clinical Data Management (CDM) department to on generating more data checks since it is easy to copy a single edit check macro .In the fast-paced world of clinical research, ensuring data quality and integrity is of utmost importance. Electronic Data Capture (EDC) systems have become indispensable for collecting, managing, and analyzing clinical trial data. One of the most critical components of EDC implementation is the design and development of Edit Check Specifications.

* Data Management Role in Clinical Research: DM Role in Clinical Research The data management function provides all data collection and data validation for a clinical trial program Data management is essential to the overall clinical research function, as its key deliverable is the data to support the submission Assuring the overall accuracy and .Edit checks are invaluable tools for increasing data quality and providing greater efficiency during data review and cleaning activities. This chapter discusses the process of edit check creation, including balance and efficiency considerations. The chapter also describes different types of edit checks, edit check validation, strategies for .Clinical data management (CDM) is a critical process in clinical research, which leads to generation of high-quality, . Edit checks are used to fire a query message when discrepant data is entered, to map certain data points from one CRF to the other, to calculate certain fields like Subject's Age, BMI etc.. Edit checks help the investigators .
Clinical Data Management: eCRF & Edit Checks Overview of edit check documentationThe following three levels of data checks should be performed: general clinical data checks, CDISC standard domain checks, and protocol compliance checks. (Doles, 2004) A. Key Variables - All unique key variables in each data set are required. 1. Demographics (DM): Subject identification number is non-missing and unique.
Drawings will be conducted on October 28, 2024. All participants will be notified of results via email and text by October 29, 2024. The event will take place in stores on December 14, 2024. View the new times and locations here . The Alabama ABC will hold a Sweepstakes to determine the top 150 places in line at a Limited Release event.The Heroic Life Of A Billionaire Security Guard | English Movies , English Drama . Show less. Recommended. 1:09:48. I. Up next. Fall Into Sweet Trap. Blue Lagoon. 1:02:15. Spoiled By My Step Brothers - Full Movie. Blue Lagoon. 1:21:43. My Fated Alpha Rejected and Reclaimed Full Movie. Blue Lagoon. 1:35:24.
edit checks in clinical data management ppt|Clinical Data Management: eCRF & Edit Checks